SARS-CoV-2 Omicron subvariant immune-evasion characterization, RBD evolution pattern prediction, and broad-spectrum neutralizing antibody drug development
2023-01-18
Collaborating institutions: Peking university, Sinovac Research & Development Co., Ltd., National Institutes for Food and Drug Control
Revealing the antibody escape mechanisms of Omicron
A high-throughput deep mutational scanning (DMS) technology was developed and a platform for high-throughput antibody selection and characterization was set up. Over 3,000 monoclonal antibodies were isolated from individuals with various SARS-CoV-2 immune histories. The neutralizing activity of these antibodies against different SARS-CoV-2 variants, and their binding sites on the receptor-binding domain (RBD) were determined by DMS. In December 2021, within 14 days after Omicron being alarmed by the WHO, we revealed that Omicron could render most neutralizing antibody drugs ineffective and cause a substantial reduction in vaccine-induced neutralization titers 3.
Reporting the mechanisms behind convergent Omicron RBD evolution
Upon the global circulation of BA.4/5 lineage, SARS-CoV-2 keeps evolving, giving rise to multiple variants with high growth advantages over BA.5. Despite their divergent evolutionary courses, these variants appear to harbor mutations on certain hotspots on RBD, such as R346, K356, K444, V445, G446, N450, L452, N460, F486, F490, R493 and S494, demonstrating a trend of “convergent evolution”.
By examining the epitope distribution of antibodies from people with different immune histories, it was found that immune imprinting led to reduced diversity of effective neutralizing antibodies elicited after Omicron breakthrough infection, which in turn focused humoral immune pressure and promoted convergent evolution 4.
Predicting SARS-CoV-2 evolution
Based on the data from neutralization assays and escape mutation profiles of a large collection of monoclonal antibodies, a computational model was built to predict the direction of virus evolution. It was found that BA.4/BA.5 can escape antibodies induced by BA.1 infection and vaccination, and that BA.2.75 and BA.5 subvariants can cause varied degrees of evasion from humoral immunity elicited by BA.5 breakthrough infection. Based on these observations, we were able to prospectively alert the global prevalence of BA.4/5 and BA.2.75. Related work was well-received by the international community 5-7.
Based on escaping mutation profiles of thousands of neutralizing antibodies, we identified critical sites for mutation that could escape neutralizing antibodies elicited by BA.5 breakthrough infection, thus the trend of RBD evolution after the global BA.4/BA.5 wave was predicted, which were later verified by emerging BA.4/5 and BA.2.75 subvariants in the real world 4.
Identifying SA55 & SA58, a pair of broad-spectrum sarbecovirus-neutralizing antibodies
A pair of neutralizing antibodies, SA55 and SA58, were isolated from SARS-CoV-2 vaccinated SARS convalescents, which can non-competingly bind to conserved epitopes on RBD 4,8. Particularly, SA55 can effectively neutralize all major circulating variants. SA55 binds to an epitope that is rarely targeted by existing antibodies elicited by current human immune background, which grants its exceptional neutralization breadth. Half-life prolonged SA55 can be used for both prophylactic and therapeutic purposes. When administered as nasal spray, it can provide short-term protection; if administered via muscular or intravenous injection, it can be used for long-term prevention and post-infection treatment. It is particularly suitable for vulnerable populations such as the elderly and immune-compromised individuals who are not fit for vaccination. SA55 is now undergoing clinical development by SinoVac. It has demonstrated high therapeutic/prophylactic efficacy and good safety profile in compassionate usage.

Fig.1 RBD convergent evolution of Omicron subvariants
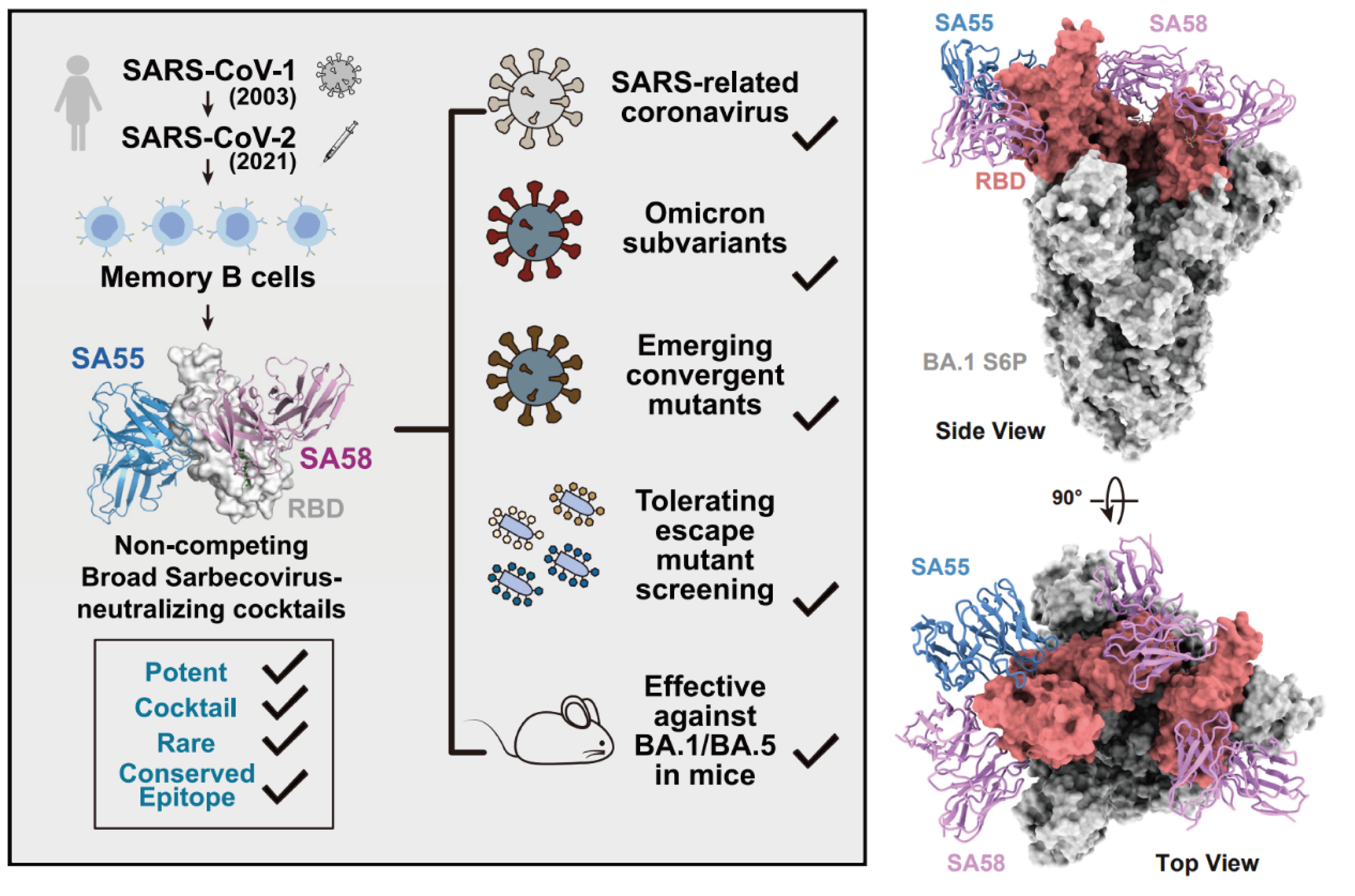
Fig.2 Broadly neutralizing antibodies SA55 and SA58
References
1.Cao, Y. et al. Humoral immune response to circulating SARS-CoV-2 variants elicited by inactivated and RBD-subunit vaccines. Cell Research 31, 732-741, doi:10.1038/s41422-021-00514-9 (2021).
2.Cao, Y. et al. Humoral immunogenicity and reactogenicity of CoronaVac or ZF2001 booster after two doses of inactivated vaccine. Cell Research 32, 107-109, doi:10.1038/s41422-021-00596-5 (2022).
3.Cao, Y. et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature 602, 657-663, doi:10.1038/s41586-021-04385-3 (2022).
4.Cao, Y. et al. Imprinted SARS-CoV-2 humoral immunity induces convergent Omicron RBD evolution. Nature, doi:10.1038/s41586-022-05644-7 (2022).
5.Cao, Y. et al. BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection. Nature 608, 593-602, doi:10.1038/s41586-022-04980-y (2022).
6.Cao, Y. et al. Characterization of the enhanced infectivity and antibody evasion of Omicron BA.2.75. Cell Host Microbe 30, 1527-1539.e1525, doi:10.1016/j.chom.2022.09.018 (2022).
7.Jian, F. et al. Further humoral immunity evasion of emerging SARS-CoV-2 BA.4 and BA.5 subvariants. The Lancet Infectious Diseases 22, 1535-1537, doi:10.1016/S1473-3099(22)00642-9 (2022).
8.Cao, Y. et al. Rational identification of potent and broad sarbecovirus-neutralizing antibody cocktails from SARS convalescents. Cell Reports, 111845, doi:https://doi.org/10.1016/j.celrep.2022.111845 (2022).